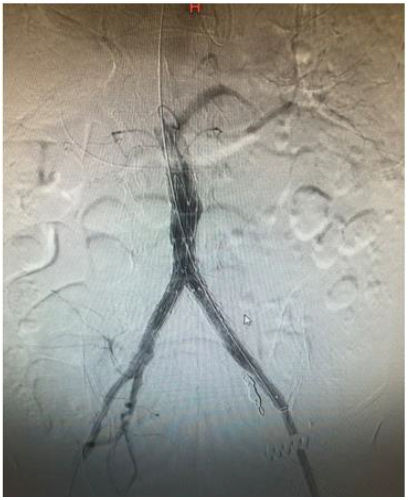
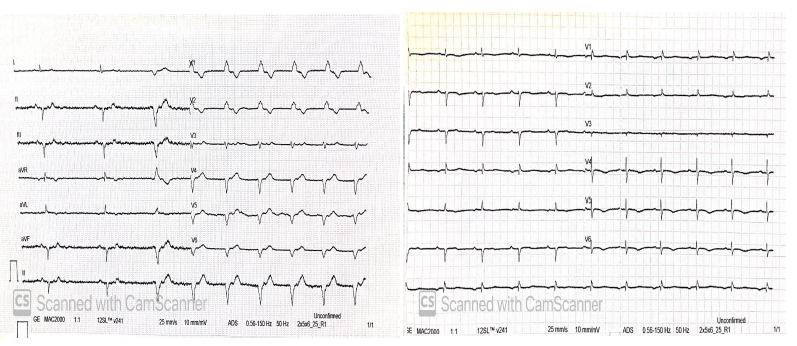
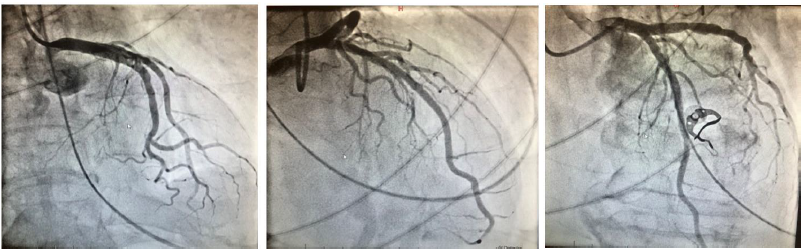
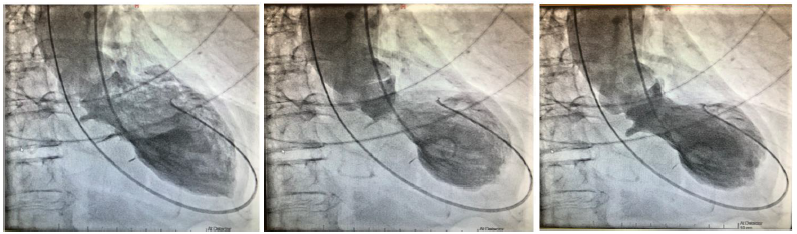
References
1. Nef, H.M., et al., Mechanisms of stress (Takotsubo) cardiomyopathy. Nature Reviews Cardiology, 2010. 7(4): p. 187-193.
2. Amin, H.Z., L.Z. Amin, and A. Pradipta, Takotsubo Cardiomyopathy: A Brief Review. J Med Life, 2020. 13(1): p. 3-7.
3. Nazir, S., et al., Takotsubo cardiomyopathy associated with epinephrine use: A systematic review and meta-analysis. Int J Cardiol, 2017. 229: p. 67-70.
4. Scantlebury, D.C. and A. Prasad, Diagnosis of Takotsubo cardiomyopathy. Circ J, 2014. 78(9): p. 2129-39.
5. Aizawa, K. and T. Suzuki, Takotsubo cardiomyopathy: Japanese perspective. Heart Fail Clin, 2013. 9(2): p. 243-7, x.
6. Sato, H., Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. Clinical aspects of myocardial injury : From ischemia to heart failure, 1990: p. 56-64.
7. Desmet, W.J., B.F. Adriaenssens, and J.A. Dens, Apical ballooning of the left ventricle: first series in white patients. Heart, 2003. 89(9): p. 1027-31.
8. Bybee, K.A., et al., Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol, 2004. 94(3): p. 343-6.
9. Pavin, D., H. Le Breton, and C. Daubert, Human stress cardiomyopathy mimicking acute myocardial syndrome. Heart, 1997. 78(5): p. 509-11.
10. Nascimento, F.O., et al., The characteristics of stress cardiomyopathy in an ethnically heterogeneous population. Clinics (Sao Paulo), 2011. 66(11): p. 1895-9.
11. Dote, K., et al., [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. J Cardiol, 1991. 21(2): p. 203-14.
12. Tsuchihashi, K., et al., Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol, 2001. 38(1): p. 11-8.
13. Pant, S., et al., Burden of arrhythmias in patients with Takotsubo cardiomyopathy (apical ballooning syndrome). Int J Cardiol, 2013. 170(1): p. 64-8.
14. Chockalingam, A., et al., Acute left ventricular dysfunction in the critically ill. Chest, 2010. 138(1): p. 198-207.
15. Pelliccia, F., et al., Pathophysiology of Takotsubo Syndrome. Circulation, 2017. 135(24): p. 2426-2441.
16. Kounis, N.G. and G.M. Zavras, Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract, 1991. 45(2): p. 121-8.
17. Ballesteros, R.V., et al., Kounis and Takotsubo, Two Syndromes Bound by Adrenaline: The “ATAK” Complex. Case Rep Cardiol, 2023. 2023: p. 7706104.
18. Kounis, N.G., et al., The ATAK complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis hypersensitivity -associated coronary syndrome) in neurological conditions. Indian J Crit Care Med, 2016. 20(4): p. 255-6.
19. Margonato, D., et al., Takotsubo cardiomyopathy associated with Kounis syndrome: A clinical case of the “ATAK complex”, in J Cardiol Cases. 2019: Japan. p. 52-56.
20. Minciullo, P.L., et al., ATAK Complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 Vaccination and Review of the Literature, in Vaccines (Basel). 2023: Switzerland.
21. Alarcón Gallardo, E., R. Escudero Apesteguía, and C. Sanz Bescós, ATAK Complex due to Amoxicillin: A Case Report. J Investig Allergol Clin Immunol, 2024. 34(1): p. 62-64.
22. Brown, S.G., R.J. Mullins, and M.S. Gold, Anaphylaxis: diagnosis and management. Med J Aust, 2006. 185(5): p. 283-9.
23. Caughey, G.H., Tryptase genetics and anaphylaxis. J Allergy Clin Immunol, 2006. 117(6): p. 1411-4.
24. Buka, R.J., et al., Anaphylaxis and Clinical Utility of Real-World Measurement of Acute Serum Tryptase in UK Emergency Departments. J Allergy Clin Immunol Pract, 2017. 5(5): p. 1280-1287.e2.
25. Pitlick, M.M. and G.W. Volcheck, Perioperative Anaphylaxis. Immunol Allergy Clin North Am, 2022. 42(1): p. 145-159.
26. Puri, P., et al., Adrenaline, Takotsubo, Anaphylaxis, and Kounis Syndrome (ATAK) Complex Unveiled: Integrating Takotsubo and Kounis Syndromes in the Context of Chemotherapy-Related Anaphylaxis. Cureus, 2024. 16(1): p. e53145.
27. Kido, K. and M. Guglin, Drug-Induced Takotsubo Cardiomyopathy. J Cardiovasc Pharmacol Ther, 2017. 22(6): p. 552-563.
28. Barmore, W., et al., Takotsubo cardiomyopathy: A comprehensive review. World J Cardiol, 2022. 14(6): p. 355-362.
29. Bairashevskaia, A.V., et al., Update of Takotsubo cardiomyopathy: Present experience and outlook for the future. Int J Cardiol Heart Vasc, 2022. 39: p. 100990.
30. Vasudev, R., et al., Selective Serotonin-norepinephrine Reuptake Inhibitors-induced Takotsubo Cardiomyopathy, in N Am J Med Sci. 2016: India. p. 312-5.
31. Jamshidi, N., D. Clark, and B. Murnion, Recurrent Takotsubo Cardiomyopathy Associated with Opioid Withdrawal During Buprenorphine Induction, in Cardiovasc Toxicol. 2021: United States. p. 349-353.
32. Saiful, F.B., et al., Takotsubo cardiomyopathy due to iatrogenic methadone withdrawal. Rev Cardiovasc Med, 2011. 12(3): p. 164-7.
33. Balsa, A.M., et al., Takotsubo Cardiomyopathy Associated with Levothyroxine Over-replacement. Eur Endocrinol, 2017. 13(1): p. 30-32.
34. Kwon, S.A., et al., A case of Takotsubo cardiomyopathy in a patient with iatrogenic thyrotoxicosis, in Int J Cardiol. 2010, Copyright © 2009 Elsevier Ireland Ltd. All rights reserved.: Netherlands. p. e111-3.
35. Laínez, B., et al., Iatrogenic tako-tsubo cardiomyopathy secondary to catecholamine administration, in Rev Esp Cardiol. 2009: Spain. p. 1498-9.
36. Pelliccia, F., et al., Takotsubo syndrome (stress cardiomyopathy): an intriguing clinical condition in search of its identity. Am J Med, 2014. 127(8): p. 699-704.
37. Ioannou, A., Iatrogenic adrenaline induced mid-ventricular Takotsubo cardiomyopathy: a case-based review. Ir J Med Sci, 2023. 192(1): p. 125-129 20. Minciullo, P.L., et al., ATAK Complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 Vaccination and Review of the Literature, in Vaccines (Basel). 2023: Switzerland.
21. Alarcón Gallardo, E., R. Escudero Apesteguía, and C. Sanz Bescós, ATAK Complex due to Amoxicillin: A Case Report. J Investig Allergol Clin Immunol, 2024. 34(1): p. 62-64.
22. Brown, S.G., R.J. Mullins, and M.S. Gold, Anaphylaxis: diagnosis and management. Med J Aust, 2006. 185(5): p. 283-9.
23. Caughey, G.H., Tryptase genetics and anaphylaxis. J Allergy Clin Immunol, 2006. 117(6): p. 1411-4.
24. Buka, R.J., et al., Anaphylaxis and Clinical Utility of Real-World Measurement of Acute Serum Tryptase in UK Emergency Departments. J Allergy Clin Immunol Pract, 2017. 5(5): p. 1280-1287.e2.
25. Pitlick, M.M. and G.W. Volcheck, Perioperative Anaphylaxis. Immunol Allergy Clin North Am, 2022. 42(1): p. 145-159.
26. Puri, P., et al., Adrenaline, Takotsubo, Anaphylaxis, and Kounis Syndrome (ATAK) Complex Unveiled: Integrating Takotsubo and Kounis Syndromes in the Context of Chemotherapy-Related Anaphylaxis. Cureus, 2024. 16(1): p. e53145.
27. Kido, K. and M. Guglin, Drug-Induced Takotsubo Cardiomyopathy. J Cardiovasc Pharmacol Ther, 2017. 22(6): p. 552-563.
28. Barmore, W., et al., Takotsubo cardiomyopathy: A comprehensive review. World J Cardiol, 2022. 14(6): p. 355-362.
29. Bairashevskaia, A.V., et al., Update of Takotsubo cardiomyopathy: Present experience and outlook for the future. Int J Cardiol Heart Vasc, 2022. 39: p. 100990.
30. Vasudev, R., et al., Selective Serotonin-norepinephrine Reuptake Inhibitors-induced Takotsubo Cardiomyopathy, in N Am J Med Sci. 2016: India. p. 312-5.
31. Jamshidi, N., D. Clark, and B. Murnion, Recurrent Takotsubo Cardiomyopathy Associated with Opioid Withdrawal During Buprenorphine Induction, in Cardiovasc Toxicol. 2021: United States. p. 349-353.
32. Saiful, F.B., et al., Takotsubo cardiomyopathy due to iatrogenic methadone withdrawal. Rev Cardiovasc Med, 2011. 12(3): p. 164-7.
33. Balsa, A.M., et al., Takotsubo Cardiomyopathy Associated with Levothyroxine Over-replacement. Eur Endocrinol, 2017. 13(1): p. 30-32.
34. Kwon, S.A., et al., A case of Takotsubo cardiomyopathy in a patient with iatrogenic thyrotoxicosis, in Int J Cardiol. 2010, Copyright © 2009 Elsevier Ireland Ltd. All rights reserved.: Netherlands. p. e111-3.
35. Laínez, B., et al., Iatrogenic tako-tsubo cardiomyopathy secondary to catecholamine administration, in Rev Esp Cardiol. 2009: Spain. p. 1498-9.
36. Pelliccia, F., et al., Takotsubo syndrome (stress cardiomyopathy): an intriguing clinical condition in search of its identity. Am J Med, 2014. 127(8): p. 699-704.
37. Ioannou, A., Iatrogenic adrenaline induced mid-ventricular Takotsubo cardiomyopathy: a case-based review. Ir J Med Sci, 2023. 192(1): p. 125-129. 38. Ong, G.J., et al., Incremental “Therapeutic” Myocardial Exposure to Catecholamines: Incidence and Impact in Takotsubo Syndrome. Cardiovasc Drugs Ther, 2020. 34(1): p. 95-100.
39. Coupez, E., et al., A single pathophysiological pathway in Takotsubo cardiomyopathy: Catecholaminergic stress. Arch Cardiovasc Dis, 2014. 107(4): p. 245-52.
40. Litvinov, I.V., M.A. Kotowycz, and S. Wassmann, Iatrogenic epinephrine-induced reverse Takotsubo cardiomyopathy: direct evidence supporting the role of catecholamines in the pathophysiology of the “broken heart syndrome”. Clin Res Cardiol, 2009. 98(7): p. 457-62.
41. Lopera, V., J.A. Pereañez, and P.J. Amariles, Drugs as Possible Triggers of Takotsubo Cardiomyopathy- Update 2022: Systematic Review. Curr Vasc Pharmacol, 2023. 21(5): p. 304-315.
42. Amariles, P. and L. Cifuentes, Drugs as Possible Triggers of Takotsubo Cardiomyopathy: A Comprehensive Literature Search – Update 2015. Curr Clin Pharmacol, 2016. 11(2): p. 95-109.
43. Arunkumar, S. and K. Jegaverrapandi, Pharmacological Triggers of Takotsubo Cardiomyopathy: An Updated Review of Evidence and Recommendations. Curr Cardiol Rev, 2024. 20(2): p. 50-60.
44. Bhat, S., et al., Catecholamine-induced reverse takotsubo cardiomyopathy, in Proc (Bayl Univ Med Cent). 2019, © 2019 Baylor University Medical Center.: United States. p. 567-569.
45. Sundbøll, J., et al., Iatrogenic takotsubo cardiomyopathy induced by locally applied epinephrine and cocaine. BMJ Case Rep, 2014. 2014.
46. Calogiuri, G., et al., Is Adrenaline Always the First Choice Therapy of Anaphylaxis? An Allergist-cardiologist Interdisciplinary Point of View. Curr Pharm Des, 2023. 29(32): p. 2545-2551.
47. Wong, C.P., et al., Iatrogenic Tako-Tsubo cardiomyopathy, in Int J Cardiol. 2008: Netherlands. p. e16-8.
48. Pathangey, G., et al., Myocardial stunning secondary to erroneous administration of intravenous epinephrine, in SAGE Open Med Case Rep. 2023, © The Author(s) 2023.: England. p. 2050313×231159732.
49. Cronin, M., D. Moradi, and P. Cotter, Angiotensin-Converting Enzyme Inhibitor-Induced Oropharyngeal edema with Subsequent Stress-Cardiomyopathy, in J Cardiovasc Echogr. 2021, Copyright: © 2021 Journal of Cardiovascular Echography.: India. p. 184-186.
50. Khoueiry, G., et al., Reverse Takotsubo cardiomyopathy in the setting of anaphylaxis treated with high-dose
intravenous epinephrine. J Emerg Med, 2013. 44(1): p. 96-9.
51. Kajander, O.A., et al., Iatrogenic inverted Takotsubo syndrome following intravenous adrenaline injections for an allergic reaction. Int J Cardiol, 2013. 165(1): p. e3-5.
52. Magri, C.J., S. Fava, and H. Felice, Inverted takotsubo cardiomyopathy secondary to adrenaline injection. Br J Hosp Med (Lond), 2011. 72(11): p. 646-7.
53. Collen, J., W. Bimson, and P. Devine, A variant of Takotsubo cardiomyopathy: a rare complication in the electrophysiology lab. J Invasive Cardiol, 2008. 20(11): p. E310-3.
54. Chen, Y.H., et al., Iatrogenic Takotsubo Cardiomyopathy Following Overdose Norepinephrine Administration During Percutaneous Coronary Intervention. Int Heart J, 2020. 61(6): p. 1298-1302.
55. Vieira, A., B. Batista, and T.T. de Abreu, Iatrogenic Takotsubo Cardiomyopathy Secondary to Norepinephrine by Continuous Infusion for Shock. Eur J Case Rep Intern Med, 2018. 5(7): p. 000894.
56. Ouerghi, K., et al., Reverse iatrogenic Takotsubo syndrome after accidental bolus of norepinephrine in the setting of sepsis. Kardiol Pol, 2016. 74(8): p. 799.
57. Madias, J.E., Iatrogenic or Anaphylaxis-Triggered Takotsubo Syndrome? Am J Ther, 2018. 25(6): p. e735.
58. Cha, Y.S., et al., Evaluation of myocardial injury through serum troponin I and echocardiography in anaphylaxis. Am J Emerg Med, 2016. 34(2): p. 140-4.
59. Kounis, N.G., et al., Kounis syndrome: a new twist on an old disease. Future Cardiol, 2011. 7(6): p. 805-24.
60. Mert, G., et al., Takotsubo cardiomyopathy or Kounis syndrome or both? Int J Cardiol, 2015. 179: p. 16.
61. Kotsiou, O.S., K.I. Xirogiannis, and K.I. Gourgoulianis, Kounis Syndrome: Is it Really a Takotsubo-Like Syndrome? J Investig Allergol Clin Immunol, 2017. 27(3): p. 198-200.
62. Ogaz, T.A. and B. Sweitzer, Intraoperative Kounis Syndrome and Fixation Errors: A Case Report. A A Pract, 2023. 17(3): p. e01672.
63. Di Filippo, C., et al., Kounis Syndrome Associated With Takotsubo Syndrome in an Adolescent With Peutz-Jeghers Syndrome, in JACC Case Rep. 2021, © 2021 The Authors.: Netherlands. p. 1602-1606.
64. Yanagawa, Y., et al., A case of takotsubo cardiomyopathy associated with Kounis syndrome, in Int J Cardiol. 2009: Netherlands. p. e65-7.
65. Kounis, N.G., et al., “When,” “Where,” and “How” of SARS-CoV-2 Infection Affects the Human Cardiovascular System: A Narrative Review. Balkan Med J, 2024. 41(1): p. 7-22.
 |
|  ISSN: 2997-321X Doi: 10.47991/2997-321X/AJCRR-114
ISSN: 2997-321X Doi: 10.47991/2997-321X/AJCRR-114