 |
|  ISSN: 2997-321X Doi: 10.47991/2997-321X/AJCRR-1106
ISSN: 2997-321X Doi: 10.47991/2997-321X/AJCRR-1106
Thrombolysis in The First Trimester of Pregnancy in Patent Foramen Ovale Related Ischemic Stroke
(Running title: Thrombolysis in PFO related ischemic stroke during pregnancy)
Author(s): Haider Faeq Hadhratee Al-Rubaiee1*, Stine Bang Kjelgaard1, Annabella Obál2, Lorenz Oppel1, Boris Modrau1, Izabella Obál1
1Department of Neurology, Aalborg University Hospital, Aalborg, Denmark
2Department of Behavioral Sciences, University of Szeged, Szeged, Hungary
Haider Alrubaiee
Department of Neurology, Aalborg University Hospital, Aalborg, Denmark. Tel: +4581901403 email: h.alrubaiee@rn.dk
Citation: Alrubaiee H, Kjelgaard SB, Obál A, Oppel L, Modrau B, Obál I (2024) Thrombolysis in The First Trimester of Pregnancy in Patent Foramen Ovale Related Ischemic Stroke. American J Cas Rep Rev: AJCRR-110.
Received: 19 June, 2024
Accepted: 24 June, 2024
Published: 30 June, 2024
Abstract
Background: Patent foramen ovale is described to be responsible for a high percent of ischemic stroke in young adults. Furthermore, ischemic stroke is considered to be one of the main pregnancy-related risk factors, contributing to maternal death. Minor stroke is however successfully managed with dual antiplatelet treatment, but presence of vascular occlusion represents a management challenge.
Case presentation: We describe the case of a 29-year-old woman, who presented with an acute onset ischemic stroke with facial weakness and sensory symptoms in the left arm. Imaging showed signs of an embolic stroke with a distal middle cerebral artery occlusion not eligible for thrombectomy. The patient was successfully treated with thrombolysis. Diagnostic work-up revealed a considerable patent foramen ovale, which was occluded 13 months after delivery. The use of the PASCAL and ROPE-score is discussed to grade the clinical relevance of the patent foramen ovale in combination with the procoagulant state during pregnancy in our patient.
Conclusion: Patent foramen ovale should be taken into account in ischemic stroke occurring during pregnancy in young women, especially in the first trimester. Possible further progression can be considered during the treatment decision regarding thrombolysis and/or endovascular intervention even in patients with a low NIHSS score.
Keywords: ischemic stroke, PFO, thrombolysis, pregnancy.
Abbreviations
AIS: acute ischemic stroke
DVT: deep venous thrombosis
DWI: diffusion weighted imaging
D2-test: measure of selective and sustained attention and visual scanning speed
ECG: Electrocardiogram
EVT: mechanical endovascular thrombectomy
FLAIR: fluid attenuated inversion recovery-weighted sequences
HELLP: hemolysis, elevated liver enzymes and low platelets
MRI: Magnetic Resonance Imaging
NIHSS: National institute of health stroke scale
PASCAL approach:
PFO: patent foramen ovale
RoPE score: risk of Paradoxical Embolism score
SWI: susceptibility weighted imaging
SLE: systemic lupus erythematosus
tPA: tissue plasminogen activator
WAIS-IV-digit span: tests working memory, mental manipulation, cognitive flexibility, rote memory and learning, attention, and encoding
Background
Acute ischemic stroke (AIS) and hemorrhagic stroke during pregnancy and puerperium occur rarely but can present with severe complications and increased risk of morbidity and mortality. According to data, the incidence of maternal stroke in the United States of America is estimated to be 30 in 100,000 pregnancies [1], while the mortality rate is 7.4% [2].
Numerous studies have shown thrombolysis with intravenous recombinant tissue plasminogen activator (tPA) as an effective therapy for AIS, when given in the first 4.5 hours after symptom onset [3]. However, information on pregnant patients is limited, as pregnancy had been an exclusion criterion in earlier trials. The American Heart Association states that tPA administration may be considered in pregnancy when the anticipated benefits of treating moderate or severe stroke outweigh the anticipated increased risks of uterine bleeding [3]. The 2022 expert consensus of the European Stroke Organization advises tPA treatment for pregnant women with acute disabling ischemic stroke, who otherwise meet eligibility criteria after appropriately assessing the benefit/risk profile on an individual basis [4]. Until December 2023 no cases of possible teratogenicity have been documented, which is in line with data from animal studies that showed that tPA does not cross the placenta.
Furthermore, minor stroke, defined as often defined by the severity of neurological deficit, size of ischemic lesion(s) on neuroimaging, and level of disability. The definition of minor stroke is often tangled with that of transient ischamic attack (TIA). The general consensus is that minor stroke is defined by NIHSS less or equal to 3. (29).
Furthermore, expert consensus suggests mechanical endovascular thrombectomy (EVT) in the case of large vessel occlusion after appropriate assessment of the benefit/risk profile on an individual basis [4].
Case Presentation
A 29- year- old, right-handed healthy woman gravida 2, para 1, gestation week 7, was admitted to the stroke unit with acute onset of left sided facial weakness and altered sensation in the left arm. The patient arrived 40 minutes after the onset of symptoms. Neurological examination confirmed a left sided, central facial nerve palsy and hypoesthesia in the left arm (NIHSS 2). Blood pressure upon arrival was 150/90 mmHg. Initial laboratory studies revealed normal peripheral cell counts, coagulation parameters and electrolyte levels. ECG showed sinus rhythm without ischemic changes or signs of conduction disturbances. Magnetic resonance imaging (MRI) of the brain with diffusion weighted imaging (DWI) showed acute ischemic stroke in the territory of the right middle cerebral artery (distal M3-segment) (Figure 1. A). No signal changes were visible on fluid attenuated inversion recovery-weighted sequences (FLAIR) (Figure 1. B). No hemorrhage, but a thrombus was visible in the relevant, distal M2-segment (Figure 1. C).
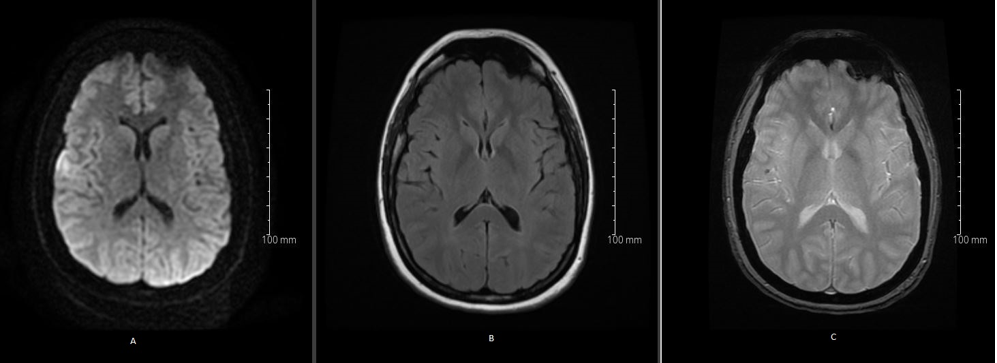
Figure 1: Magnetic resonance imaging (MRI) of the brain with diffusion weighted imaging (DWI) showed acute ischemic stroke in the territory of the right middle cerebral artery (distal M3-segment) (A). No signal changes were observed on fluid attenuated inversion recovery-weighted sequences (FLAIR) (B). Susceptibility weighted imaging (SWI) did not reveal hemorrhage, but a thrombus was visible in the relevant, distal M2-segment (C).
The patient’s situation was reviewed, and several factors were considered:
• M2 thrombus not feasible for EVT
• Imaging with an embolic ischemic stroke pattern of unknown etiology
• Risk of thrombus growth and/or further embolization
• Severity of stroke at the timepoint of assessment (non-disabling minor stroke with NIHSS 2)
Despite the low NIHSS score, the presence of an M2 thrombus was evaluated to be associated with a worse prognosis and with a possibility of progression, therefore tPA treatment was offered. The patient accepted, informed consent was obtained, and routine tPA treatment with total dose of 72 mg was administered, 7 mg over 2 minutes and 65 mg over 1 hour.
Neurological improvement could be observed after 24 hours with only a slight residual central facial palsy remaining (NIHSS 1). Minor vaginal bleeding was detected 10 hours after tPA treatment. Acute sonographic and gynecological examination showed normal gestational sac and fetus.
Antiplatelet therapy with 75 mg acetylsalicylic acid was initiated. Control MRI of the brain 24 hours after tPA treatment showed infarct demarcation on DWI (Figure 2. A), with a hyperintense signal on FLAIR-sequences (Figure 2. B). No hemorrhagic transformation was seen on the SWI-sequence. The M2 thrombus was still visible after thrombolysis (Figure 2. C). Ultrasound scan of the carotid and vertebral arteries showed normal hemodynamic state and no signs of atherosclerosis or dissection. Control electrocardiography as well as a 5-day cardiac monitoring showed sinus rhythm, and could not detect any signs of arrythmia, especially no atrial fibrillation. Routine blood tests including D-dimer and additional work up for thrombophilia were normal, transthoracic echocardiography raised suspicion of a patent foramen ovale (PFO), confirmed by transesophageal echocardiography. A PFO with a size of 1.8 cm was detected with a spontaneous right to left shunt. An atrial septal aneurysm (ASA) could be seen. No clinical sign of deep venous thrombosis (DVT) was detected.
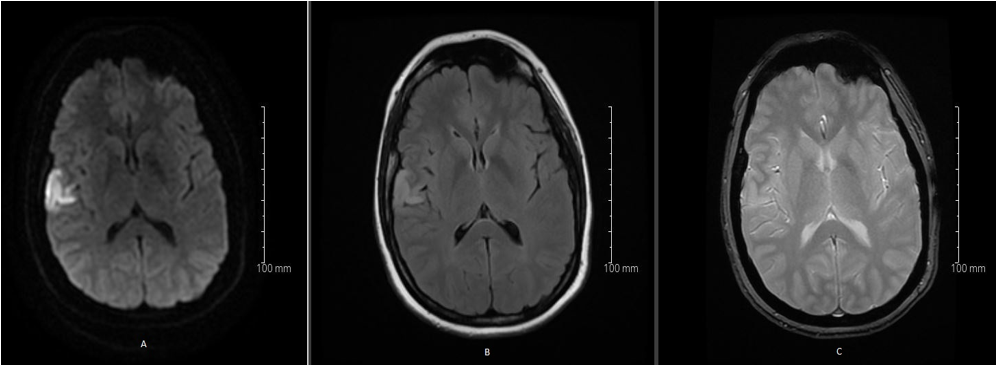
Figure 2: MRI of the brain 24 hours after tPA treatment showed infarct demarcation on DWI (A), with a hyperintense signal on FLAIR-sequences (B), without hemorrhagic transformation on the SWI-sequence. The M2 thrombus was still visible after thrombolysis(C).
The patient was discharged after 3 days. At three months follow-up, the neuropsychological examination, including an estimate of premorbid intellectual functioning, processing speed, attention, concentration, memory, visuospatial and constructional skills, and executive functions was carried out. Overall, most of the test performances were at or above the average level. However, single failures on text encoding, WAIS-IV Digit Span and on the d2-test did indicate mild attention deficits. No specific treatment was required.
During pregnancy, the gynecological follow up was unremarkable, and the patient had an uncomplicated, spontaneous vaginal delivery in week 39+4. No neonatal abnormality was detected at the time of delivery and a normal development was observed until the last follow-up, 18 months after the delivery.
The neurovascular work-up could not show any further contributing factors aside from the potential thrombogenic risk related to pregnancy and the demonstrated PFO. Occlusion of the PFO with an Amplatzer TM septal occluder (Abbott) 13 months after delivery was uneventful, and no further complications were observed on follow-up.
Discussion
This case report presents a 29-year-old woman with AIS. The patient was pregnant in the first trimester, received thrombolysis successfully with tPA, and no major maternal or fetal complications were observed. The diagnostic workup could not detect any lifestyle factors, migraine, comorbidity with autoimmune diseases, or vasculopathies. Hypertensive disorders related to pregnancy were not present previously or during the follow-up. Hereditary forms of thrombophilia and valvopathy were excluded.
Best management for minor stroke, defined as NIHSS of less or equal 3, were thoroughly investigated, with number of trials, notably ARAMIS randomized clinical trial, which found non-inferiority of dual antiplatelet treatment with clopidogrel and Aspirin comparing to IV thrombolysis [30], in regard to functional outcome after 90 days. Although no mention whether the patients also had large vessel occlusion or whether the outcome was also non-inferior in the vessel occlusion group.
Early neurological deterioration (END) after stroke is described as worsening of symptoms after acute ischemic stroke (AIS), which occurs in 5–40% of patients. The time interval in which END occurs is not standardized but is commonly described as neurological deterioration within 24–72 hours following AIS. The risk factor for it is large vessel occlusion, although cardioembolism is less associated with END, though it is diagnostic challenge in the acute setting to know whether artery occlusion is caused by large vessel disease or cardioembolism (our case) [31]. Therefore, a quick decision is always required in this setting.
Another argument, which was in our case, is that the patient is pregnant. Clopidogrel is generally class B1 in pregnancy [33], that is to say to be avoided if possible. The drug crosses the placenta and limited data on its safety in pregnancy is available [32].
As a potential embolic source, a PFO with a prominent atrial septal aneurysm and significant right to left shunt was identified. This is not unusual as PFOs are found in up to 50% of all cryptogenic strokes [5]. The cause-and-effect relationship between various PFO morphologies and ischemic stroke has been extensively investigated in the general population. Case control studies demonstrated an increase in the prevalence of PFO both in younger and older patients with cryptogenic strokes [6,7,8,9]. Moreover, a combination of PFO with atrial septum aneurysm or large shunt was shown to further increase the risk of recurrent stroke [9,10]. Atrial septum aneurysm was described to be a more significant prognostic factor than shunt size [11] in recurrent stroke. Prospective, population-based studies, however, showed different results [12, 13], some could not prove a correlation between PFO, other septal anomalies, shunting and stroke, which is probably due to the reason that most of these studies have investigated patients in the higher age groups, where other etiologies dominate [14]. Nonetheless, studies have concluded that “cryptogenic” ischemic strokes in young patients with typical embolic-appearing neuroimaging and associated PFO, complicated with a right-to-left shunt are in all probability PFO-related [15].
In our case, the echocardiography could not visualize a straddling or other type of thrombus after thrombolysis. However, the examination was not done acutely before the treatment. The size of the PFO was classified as large with the PASCAL approach [15], because of the diameter of 1,8 cm and the number of microbubbles being over 20 in connection with the injection of agitated saline contrast spontaneously. Using the risk of paradoxical embolism (RoPE) score (16), which incorporates age, vascular risk factors and cortical imaging, our patient could be evaluated as one with a pathogenic PFO (RoPE score 9).
Elgendy et al proposed a new classification system which estimates the causal association of PFO with ischemic stroke [15] (PASCAL). Our patient was presented with a typical, wedge-shaped, embolic-appearing ischemic stroke affecting the cortex and the underlying subcortical white matter with a thrombus in the M2 segment. The new clinical approach system combines the RoPE score with the clinical factors to specify the possible causative connection. Based on this clinical approach, the association in our case is “probable”. In summary, the factors listed above contributed to our assessment that the PFO fulfills the criteria for being a probable source for paradox embolism and responsible for the patient’s stroke. The pathomechanism behind the embolic ischemic stroke related to PFO is thought to be a paradoxical embolic phenomenon from venous thromboembolism, and/or thrombus related to the PFO or ASA. Many cases are documented with deep venous thrombosis, paradoxical embolism, and PFO-related stroke in the general population [17,18]. Pregnant women have a further fivefold increased risk to develop venous thromboembolism [19]. Factors which predispose to the high risk are slow deep venous circulation, elevated hormone levels, the increase in the levels of coagulation factors V, VII, VIII, IX, X, XII and von Willebrand, as well as the plasma plasminogen activator [20,21]. These changes are accompanied by a decrease in the concentration of Protein S and C. Structural anomalies such as May-Thurner syndrome should also be considered [22]. In our case, hypercoagulability during pregnancy could be propagated, although indirect sign of venous thrombosis could not be detected with D dimer analysis. The possibility that a thrombus in the extracerebral circulation was dissolved by the thrombolysis cannot be excluded. In fact, previous studies have described thrombolysis to be effective in the treatment of proximal pelvic vein thrombi in cryptogenic stroke [23,24].
Literature is sparse regarding PFO-related stroke in pregnant women. PFO induced embolic stroke is probably underreported, which is likely, because PFO as a sole causative agent has been much argued in the past. As opposed to other stroke causes, PFO-related stroke develops mostly during early pregnancy, in the first and second trimester and can have a better outcome. A review from 2016 examined the period between 1970 and 2015 and summarized 16 cases in the literature with PFO-related ischemic stroke during pregnancy [5]. 60% of cases occurred in the first two-thirds of the pregnancy, 20% postpartum [5]. Only one patient was treated with intraarterial thrombolysis with good effect [25] and no bleeding complications, who also had an additional comorbidity of a pulmonary AVM with recurrent ischemic stroke. Many of the patients reported in the review received aspirin, warfarin, or heparin before PFO closure. It should be emphasized, that the cases described, are from a period when thrombolysis was rarely done in pregnant women due to lack of evidence.
In a case from 2019 a “thrombus in transit” was detected in a pregnant woman with DVT. Paradoxical embolism however was not visible, and the thrombus was surgically removed [26]. In the event of a thrombus trapped in a PFO a multidisciplinary approach is needed for optimal diagnostics and treatment [27]. Data shows that PFO closure is superior to antiplatelet therapy to prevent recurrent strokes [15,28]. Therefore, the occlusion of the PFO was warranted during the follow up of our patient.
Our case highlights the fact that PFO should be considered in the context of ischemic stroke in young pregnant women without other comorbidities. Despite a low NIHSS score, thrombolysis can be a treatment option, if there is a risk of paradoxical embolism and thereby recurrent embolic stroke with further progression.
Conclusion
This case report describes successful thrombolysis in a pregnant woman with AIS and distal middle cerebral artery occlusion not eligible for thrombectomy. The long-term follow-up for both the mother and the child was excellent. The use of the PASCAL and ROPE-score was discussed to grade the clinical relevance of the PFO in combination with the procoagulant state during pregnancy in our patient.
Declarations
Ethics Approval and Consent to participate: not applicable
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent for publication: Written consent was obtained from the patient regarding publication of data and pictures.
Availability of data and materials: All data generated or analyzed during this study are included in this published article [and its supplementary information files]
Competing interests: The authors declare that they have no competing interests.
Funding: This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors` contributions: IO examined, diagnosed and treated the patient. HFHAR collected data and summarized the literature. SBK performed the neuropsychological examination. AO contributed to the evaluation of the neuropsychological examination and literature search. HFHAR, AO, LO, BM and IO were responsible for the drafting of the article.
Acknowledgement: not applicable
Reference
Swartz RH, Cayley ML, Foley N, Ladhani NNN, Lefert L, Bushnell C, McClure JA,et al. The incidence of pregnancy-related stroke: a systematic review and meta-analysis. Int J Stroke. 2017;12 (7)
Elgendy IY, Bukhari S, Barakat AF, Pepine CJ, Lindley KJ, Miller EChttps://pubmed.ncbi.nlm.nih.gov/33587666/ et al. Maternal Stroke: A Call for Action. 2021;143 (7)
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke. Stroke 2019;50 (12)
Kremer C, Gdovinova Z, Bejot Y, Heldner MR, Zuurbier S, Walter S, et al. European Stroke Organisation guidelines on stroke in women; Management of menopause, pregnancy and postpartum Eur Stroke J. 2022;7 (2)
Chen L, Deng W, Palacios I, Inglessis-Azuaje I, McMullin D, et al. Patent foramen Ovale (PFO), stroke and pregnancy. Investig Med. 2016;64 (5).
Mazzucco S, Li L, Binney L, Rothwell PM; Oxford Vascular Study Phenotyped Cohort. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol. 2018;17 (7).
Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357(22).
Di Tullio M, Sacco RL, Gopal A, Homma S et al. Patent foramen ovale as a risk factor for cryptogenic stroke Ann Intern Med. 1992;117 (6).
Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. 1993;24 (12).
Mattioli AV, Aquilina M, Oldani A, Longhini C, Mattioli G. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J. 2001;22 (3).
Turc G, Lee JY, Brochet E, Kim JS, Song JK, Mas JL; CLOSE and DEFENSE-PFO Trial Investigators Atrial Septal Aneurysm, Shunt Size, and Recurrent Stroke Risk in Patients with Patent Foramen Ovale. J Am Coll Cardiol. 2020;75 (18).
Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. 2002;33 (3).
Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345 (24).
Serena J, Marti-Fàbregas J, Santamarina E, Rodríguez JJ, Perez-Ayuso MJ, Masjuan J. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. 2008;39 (12).
Elgendy AY, Saver JL, Amin Z, Boudoulas KD, Carroll JD, Elgendy IY et al. Proposal for updated nomenclature and classification of potential causative mechanism in patent foramen ovale associated stroke. JAMA Neurol. 2020;77 (7).
Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. 2013;81 (7)
Mascarenhas V, Kalyanasundaram A, Nassef LA, Lico S, Qureshi A. Simultaneous massive pulmonary embolism and impending paradoxical embolism through a patent foramen ovale. J Am Coll Cardiol 2009;53 (15).
Lethen H, Flachskampf FA, Schneider R, Sliwka U, Köhn G, Noth J. Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack Am J Cardiol. 1997;80 (8).
Gersony DR, Kim SH, Di Tullio M, Fard A, Rabbani L, Homma S. Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale. J Am Soc Echocardiogr. 2001;14 (12).
Macklon NS, Greer IA, Bowman AW. An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol. 1997; 104 (2).
Greer I. Haemostasis and thrombosis in pregnancy. In: Bloom AL, Forbes CD, Thomas DP, et al., Hemostasis and thrombosis. Edinburgh: Churchill Livingstone; 1994. p.987–1015.
Bai H, Cho LD, Cooke PV, Windsor T. Endovascular Intervention for May-thurner Syndrome in a Pregnant Patient with a Patent Foramen Ovale and Paradoxical Embolism. Vasc Endovascular Surg. 2022;56 (5)
Cramer SC, Rordorf G, Maki JH, Kramer LA, Grotta JC, Burgin WS. Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) study. Stroke 2004; 35 (1).
Nakamura S, Tokunaga T, Yamaguchi A, Kono T, Kasano K, Yoshiwara H. Paradoxical embolism caused by ovarian vein thrombosis extending to inferior vena cava in a female with uterine myoma. J Cardiol Cases. 2018;18 (6).
Li Y, Margraf J, Kluck B, Jenny D, Castaldo J. Thrombolytic therapy for ischemic stroke secondary to paradoxical embolism in pregnancy: a case report and literature review Neurologist. 2012; 18 (1)
Nan J, Tan N, Schaff H, Bell MR, Pislaru S, Best PJM. A Dangerous Dilemma: Thrombus in transit during Pregnancy JACC Case Rep. 2019;1 (3).
Bhat V, Lane S, Orde S. Management of straddling thrombus through patent foramen ovale complicating pulmonary embolism. BMJ Case Rep. 2021;14 (4)
Kneihsl M, Horner S, Hatab I, Schöngrundner N, Kramer D, Toth-Gayor G. et al. Long-term risk of recurrent cerebrovascular events after patent foramen ovale closure: Results from a real-world stroke-cohort. Eur Stroke J 2023;8 (4).
Leng X, Wang DEditorial: minor stroke is not minorStroke and Vascular Neurology 2023;8: doi: 10.1136/svn-2022-002049
Chen H, Cui Y, Zhou Z, et al. Dual Antiplatelet Therapy vs Alteplase for Patients with Minor Nondisabling Acute Ischemic Stroke: The ARAMIS Randomized Clinical Trial. 2023;329(24):2135–2144. doi:10.1001/jama.2023.7827
Sabir Rashid A, Huang-Link Y, Johnsson M, Wetterhäll S, Gauffin H. Predictors of Early Neurological Deterioration and Functional Outcome in Acute Ischemic Stroke: The Importance of Large Artery Disease, Hyperglycemia and Inflammatory Blood Biomarkers. Neuropsychiatr Dis Treat. 2022 Sep 6;18: 1993-2002. doi: 10.2147/NDT.S365758. PMID: 36097537; PMCID: PMC9464020.
De Santis M, De Luca C, Mappa I, Cesari E, Mazza A, Quattrocchi T, Caruso A. Clopidogrel treatment during pregnancy: a case report and a review of literature. Intern Med. 2011;50(16):1769-73. doi: 10.2169/internalmedicine.50.5294. Epub 2011 Aug 15. PMID: 21841343.
Drugs.com
